State Key Laboratory of Medicinal Chemical Biology
Enrichment
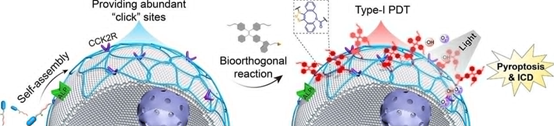
of photosensitizers (PSs) on cancer cell membranes via bioorthogonal reactions
is considered to be a very promising therapeutic modality. However,
azide-modified sugars-based metabolic labeling processes usually lack targeting
and the labeling speed is relatively slow. Moreover, it has been rarely
reported that membrane-anchoring pure type-I PSs can induce cancer cell
pyroptosis. Here, we report an alkaline phosphatase (ALP) and cholecystokinin-2
receptor (CCK2R) dual-targeting peptide named DBCO-pYCCK6, which can
selectively and rapidly self-assemble on cancer cell membrane, and then
bioorthogonal enrich type-I aggregation-induced emission luminogens (AIEgen)
PSs (SAIE-N3) on the cell membrane. Upon light irradiation, the
membrane-anchoring SAIE-N3 could effectively generate type-I reactive oxygen
species (ROS) to induce gasdermin E (GSDME)-mediated pyroptosis. In vivo
experiments demonstrated that the bioorthogonal combination strategy of peptide
and AIEgen PSs could significantly inhibit tumor growth, which is accompanied
by CD8+ cytotoxic T cell infiltration. This work provides a novel self-assembly
peptide-mediated bioorthogonal reaction strategy to bridge the supramolecular
self-assembly and AIE field through strain-promoted azide-alkyne cycloaddition
(SPAAC) and elucidates that pure type-I membrane-anchoring PSs can be used for
cancer therapy via GSDME-mediated pyroptosis.
 津公网安备 12010402001780号
津公网安备 12010402001780号








